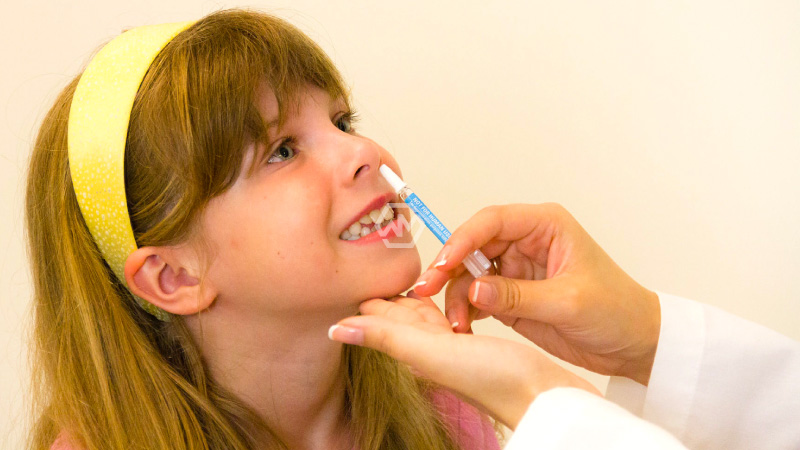
The world’s first nasal immunization for Coronavirus created by India has got an endorsement from the Focal Medications Standard Control Association (CDSCO) for confined use in crisis circumstances in the age gathering of 18 or more.
This was expressed here today by the Association Priest of State (Free Charge) Science and Innovation; Clergyman of State (Free Charge) Studies of the planet; MoS PMO, Faculty, Public Complaints, Benefits, Nuclear Energy and Space, Dr. Jitendra Singh while leading the gathering of the Social orders of Independent Foundations of Branch of Biotechnology where he likewise educated about the notable choice to combine the 14 social orders of Biotechnology Organizations into a solitary society in light of a legitimate concern for helpful working, cost-viability, and coordinated working.
COVID Nasal Vaccines
The Priest commended the job of the Division of Biotechnology (DBT) and its PSU, Biotechnology Industry Exploration Help (BIRAC) for supporting the improvement of the world’s most memorable Intranasal immunization for Coronavirus by Bharat Biotech Global Restricted (BBIL).
This immunization got endorsement under limited Use in crisis circumstances for a very long time 18 or more for essential 2 portion plan, homologous promoter dosages.
Dr. Jitendra Singh said India’s endeavors through Mission Coronavirus Suraksha under the unique initiative of Top state leader Narendra Modi has reinforced AtmaNirbhar Bharat as well as supported India’s status as an overall immunization improvement and assembling focus displaying the strength of Science and Innovation. It involves incredible pride for the country, the Pastor added.
Stage III preliminaries were directed for security, and immunogenicity in ~3100 subjects, in 14 preliminary locales across India (upheld by BIRAC). Heterologous supporter portion reads were directed for security and immunogenicity in ~875 subjects, where a sponsor portion (third portion) of BBV154 intranasal immunization was managed to concentrate on members who were recently immunized with authorized Coronavirus antibodies.
- The clinical preliminaries were led in 9 preliminary locales across India.
- Dr. Jitendra Singh says that this clinical improvement was supported by a branch of the biotechnology administration in India.
- This project was also supported by BIRAC under the mission Coronavirus Suraksha Program.
Public Organization of Immunology (NII), an independent foundation of DBT in New Delhi used their “Human Resistant Observing and Lymphocyte Immunoassay Stage” to look at the immunization actuated SARS-CoV-2-explicit fundamental and mucosal cell safe reactions of the preliminary members.
Intuitive Exploration School for Wellbeing Issues (IRSHA), Pune (upheld by BIRAC) finished the “Plaque Decrease Balance Measure” (PRNT) to evaluate the titer of killing immunizer for the infection from three preliminary destinations.
This antibody has the twofold advantage of empowering quicker improvement of variation explicit immunizations and simple nasal conveyance that empowers mass inoculation to safeguard from arising variations of concern. It vows to turn into a significant apparatus in mass immunizations during pandemics and endemics.
Immunization is a recombinant replication of inadequate adenovirus vector immunization with a pre-combination settled spike protein. This antibody up-and-comer was assessed with successes, in Stages I, II, and III clinical preliminaries. It has been explicitly formed to permit intranasal conveyance through nasal drops.
The nasal conveyance framework has been planned and created to be practical in low-and center pay nations. This immunization is steady at 2-8°C for simple capacity and appropriation. Bharat Biotech has laid out enormous assembling abilities at different locales across India, including Gujarat, Karnataka, Maharashtra, and Telangana, with activities in container India.